Molly, MDMA And Ecstasy: Whats The Difference?
Are you confused about the differences between Molly, Ecstasy, and MDMA? You're not alone. These terms are often used interchangeably in both the media and party culture. However, they aren't exactly the same. In this article, we break down what each substance is, how they differ, and how you can be sure you're getting the real deal.
Molly, MDMA, and Ecstasy are three words that are often used interchangeably, both by those whose weekend partying revolves around one of these substances, and by the media. But are they all technically the same? Wonder no more as we delve into all you need to know about these substances.
Molly, MDMA & Ecstasy: What does it all mean?
As mentioned, many believe these terms are interchangeable and describe the same substance—but it’s not as straightforward as that. While all are derived from synthetic chemicals, there are notable differences between them. With that in mind, let's bring you up to speed and explain what each substance entails.

What is Ecstasy?
Otherwise known as “E” or “X”, Ecstasy is an amphetamine-based stimulant containing several varying ingredients. Ecstasy impacts the body in a wide variety of ways, and even has the potential to produce some mild hallucinogenic effects. Broadly, Ecstasy’s effects include altered perception of time, increased body temperature, and increased physical pleasure. Ecstasy has long been used as a party drug and is typically consumed in pill, capsule, or tablet form, hence the additional ingredients used.
What is Molly?
Now we've established what Ecstasy is, how does Molly factor into the equation? Known as the powdered or crystalline version of MDMA, Molly doesn't typically contain the additional ingredients that tablet-formed Ecstasy has, and is thought of as a more “pure” form when left untouched. However, this is a rare occurrence because of its consistency; Molly is usually cut with a wide variety of other substances, such as brick dust, speed, meth, heroin, chemical fertilisers, and even rat poison. All of these bulk out the substance so that a drug dealer can increase their profit without giving up more pure Molly.
What is MDMA?
Essentially, MDMA (3,4-methylenedioxymethamphetamine) is the building block of both of the aforementioned substances. But with the inclusion of other ingredients, neither can truly be called MDMA. This makes finding pure MDMA somewhat of a challenge. To give you an idea of just how unlikely it is that the drug you are taking contains any MDMA, a report from the DEA in America found that only 13% of all Molly seized actually had any MDMA content; certainly food for thought for those looking to partake.
Is there a difference in the effects?

Given the varying ingredients of each substance, the effects can differ somewhat. However, they all share one thing in common: they communicate with the neurotransmitters in the brain. These neurotransmitters are responsible for controlling reflexes, emotions, and memory, especially the neurotransmitter serotonin. Serotonin impacts your mood as well as appetite and sleep. So, when you take MDMA (in any form), it interacts with your brain to release an enormous amount of serotonin as well as other neurotransmitters, such as dopamine and norepinephrine. This, in turn, results in a massive increase in empathy, intense happiness, social feelings, and an inability to sleep, which is why it is favoured as a popular party drug.
However, while the effects are thought to last around 3–8 hours, the brain produces a huge influx of serotonin during this time, and all of it can't be used, so most of it is chemically destroyed. This means that once you “come down” and your brain begins to function normally again, there is less serotonin to bind to the receptors. This results in a hangover that can have side effects such as a negative mood, irritability, and feeling extremely tired.
What about MDA and MDEA?

While MDMA acts as the building block for Ecstasy and Molly, there are other substances that are often talked about in the same breath, such as MDA and MDEA. But what exactly are they? MDA, or 3,4-methylenedioxyamphetamine, is another synthetic substance that is chemically very similar to MDMA. In fact, MDMA is derived from MDA. However, MDA is known to cause stronger effects that lean more towards the psychedelic and hallucinogenic side, and even provide a much more euphoric and energy-boosting experience compared to MDMA.
3,4-Methylenedioxy-N-ethylamphetamine, otherwise known as MDEA or MDE, is another substance that's similar to MDMA. It was originally introduced to the recreational market in 1985 as a substitute for MDMA when it became banned in the USA. However, it's noted that the effects are much milder and shorter lasting than MDMA, but it continues to be used as an additive or as a substitute ingredient in Ecstasy pills.
Why it's important to know what you're taking
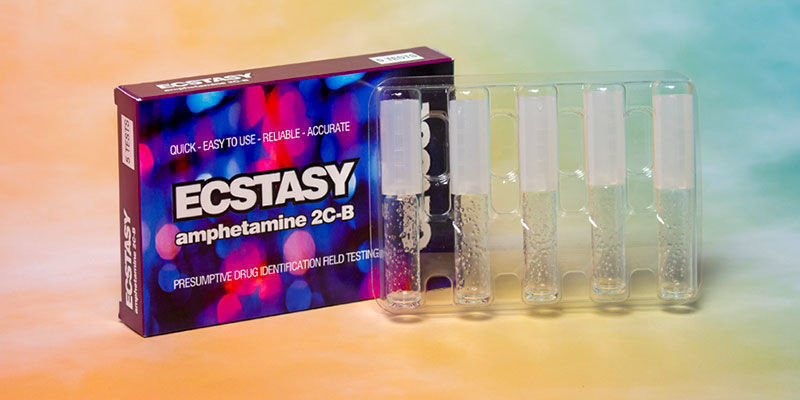
As mentioned earlier, at first glance, it's never really clear what you're truly getting when using Molly, Ecstasy, or MDMA. Although this is down to personal choice, knowing exactly what you're putting into your body is undoubtedly a good idea. Thankfully, there are a few things you can use to ascertain what's inside your pills and powder. With products such as the EZ Test Ecstasy, EZ Test MDMA Purity, and the Dope Or Nope General Drug Test, it's never been easier to figure out what you've been given. Moreover, these disposable kits are simple to use and take just minutes to provide results.
By taking a tiny sample of your chosen substance and combining it with one of these kits, it can give you an idea of what you're in for. Place around 20mg into the tube, and shake. The liquid will then change colour and give you an indication of the purity of your MDMA product. It's important to remember that Ecstasy and Molly aren't pure substances and can be cut and mixed with a variety of deadly ingredients. Putting your trust in them without testing is a fool's game. Taking that extra moment to test your substances can make all of the difference.
Ecstasy vs Molly: Knowledge is power

Ultimately, the decision of using Ecstasy or Molly is one that's entirely down to you. These substances have remained a popular party drug for decades, and it doesn't seem like that's slowing down anytime soon. Still, it's essential to put things into perspective and understand what you're putting into your body—and the potential repercussions of doing so. If you're considering trying them out, be sure to test your substances first. Fortunately, you don't have to look far for the appropriate products, as Zamnesia stocks a variety of tests that will tell you almost instantly what's in your substance.
-
 4 min
20 March 2023
Microdosing MDMA: What You Need To Know
The practice of microdosing is becoming increasingly popular, with LSD and psilocybin among the most popular substances in this context. But what about other drugs? In this article, we discuss the...
4 min
20 March 2023
Microdosing MDMA: What You Need To Know
The practice of microdosing is becoming increasingly popular, with LSD and psilocybin among the most popular substances in this context. But what about other drugs? In this article, we discuss the...
-
 4 min
16 February 2023
How To Recover From An MDMA Hangover
MDMA is famed for the glowing feelings it produces on a night out, but it can make you pay for them with a nasty hangover. Luckily, if you know how what to do, it’s possible to get right back on...
4 min
16 February 2023
How To Recover From An MDMA Hangover
MDMA is famed for the glowing feelings it produces on a night out, but it can make you pay for them with a nasty hangover. Luckily, if you know how what to do, it’s possible to get right back on...
-
 4 min
30 May 2019
Why You Should Always Test Your Drugs
Testing your drugs before using them can protect both your life and your wallet. Effectively testing your drugs can help you to determine their purity, and the trustworthiness of their source....
4 min
30 May 2019
Why You Should Always Test Your Drugs
Testing your drugs before using them can protect both your life and your wallet. Effectively testing your drugs can help you to determine their purity, and the trustworthiness of their source....
-
 5 min
1 August 2018
The MDMA Jaw Clench: What It Is And How To Stop It
Reducing MDMA jaw clench involves a combination of preemptive planning and dietary supplements.
5 min
1 August 2018
The MDMA Jaw Clench: What It Is And How To Stop It
Reducing MDMA jaw clench involves a combination of preemptive planning and dietary supplements.





 United States
United States












