What Is Cannabidiol (CBD)?

CBD has risen to stardom in the supplement world. But what propelled a single plant-derived molecule to such great heights? There are certain elements at play here. Early research has turned out some interesting findings, but marketing hype has also driven the CBD boom. Find out the facts about CBD, and what we know so far about its effects.
You’ve heard of cannabidiol (CBD) by now. These three letters can be seen plastered across billboards, in TV commercials, and on magazine covers. Today, there are many thousands of CBD products available, and consumers tend to have polarising views on the molecule. Some claim it works as a cure-all, while others believe CBD has absolutely no inherent value. But what exactly is cannabidiol, and what is it really capable of?
Below, we’ll help you navigate the hype to gain a better understanding of CBD, including where it comes from, how it works, what products are available, and if it presents any safety risks.
What exactly is CBD?

CBD, also known as cannabidiol, is a compound found in Cannabis sativa. It belongs to a chemical class known as cannabinoids, which act as secondary metabolites that defend plants against a range of stressors, from predators to UV rays.
Researchers discovered CBD back in 1941, and eventually discovered that, alongside THC and other chemical constituents, it interfaces with the human body’s innate endocannabinoid system (ECS). However, unlike THC, CBD does not cause psychotropic or intoxicating effects.
Cannabinoids, including CBD, are also classified as meroterpenes—a name given to compounds with a partial terpenoid structure. Overall, cannabinoids are part terpene and part phenol.
Where does CBD come from?

CBD occurs almost exclusively in the cannabis plant. The majority of commercial CBD in Europe is derived from fibre hemp, which features very low levels of the psychoactive constituent THC (0.2% or lower). However, medical marijuana and recreational cannabis can also contain notable levels of CBD.
If you’ve ever taken a close look at a cannabis flower, you likely noticed a shimmering layer of frosty crystals. These crystals, known as glandular trichomes, are centres of secondary metabolite production, synthesising CBD and other compounds.
In the case of CBD, it starts with two molecules: olivetolic acid and geranyl pyrophosphate. These molecules are transformed by enzymes into CBGA—the so-called “mother cannabinoid” that leads to the production of other cannabinoids. From here, the enzyme CBDA synthase converts CBGA into CBDA, the acidic precursor to CBD. The process of decarboxylation (the application of heat) ejects the carboxyl group in CBDA, resulting in CBD.
While cannabis serves as the only natural source of CBD, researchers are finding new ways to chemically synthesise CBD, often in a matter of minutes. Others have managed to create CBD by implanting the genes responsible for making the molecule into engineered brewer’s yeast.
How does CBD work?
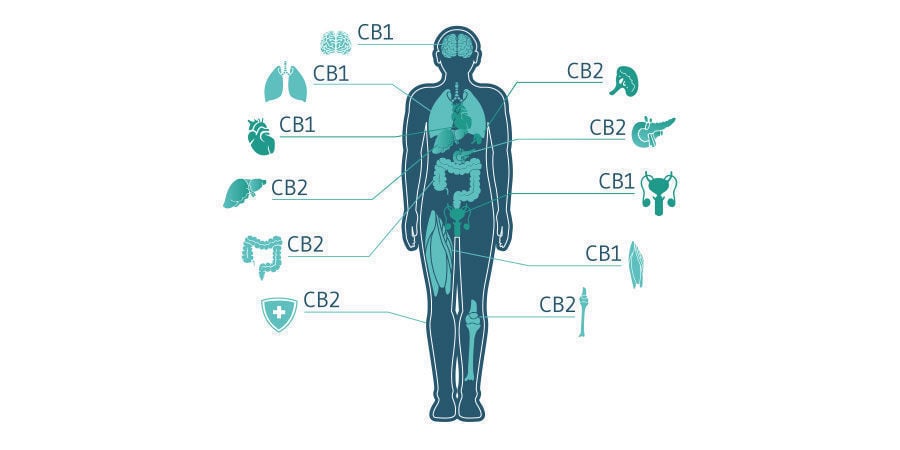
CBD works in a multifaceted manner, making it a compound with potentially far-reaching implications in humans. The compound interfaces with numerous molecular targets, including receptors of the endocannabinoid system. The ECS serves as the universal regulator of the human body, helping to govern neurotransmission, bone remodelling, skin health, immunity, and more. For this reason, researchers are particularly interested in molecules capable of influencing this system.
The ECS possesses three key components: two major receptors (known as CB1 and CB2), signalling molecules (known as endocannabinoids), and enzymes (which build and deconstruct these endocannabinoids). Because cannabis-derived cannabinoids such as THC and CBD share a similar structure to endocannabinoids, they’re able to mimic them, and therefore target the ECS in similar, but distinct, ways.
But how exactly does CBD interact with the ECS? Researchers are still trying to figure out the answer to that question. However, preliminary research has unveiled a few key clues. To start, CBD, unlike THC, doesn’t directly activate, or agonise, CB1 and CB2 receptors. Rather, it acts as a negative allosteric modulator of the CB1 receptor (Laprairie et al., 2015), meaning it can reduce receptor activation by other molecules. For example, CBD is thought to mediate the psychoactive potential of THC when taken in tandem, by blocking THC’s affinity with CB1.
Studies are also looking to determine if CBD can alter levels of circulating endocannabinoids in the body. The enzyme fatty acid amide hydrolase (aka FAAH) works by breaking down endocannabinoids after they’ve fulfilled their function. Pharmaceutical companies have developed a range of drugs, known as FAAH-inhibitors, that temporarily block the enzyme's activity, subsequently raising endocannabinoid levels. As such, researchers are looking to ascertain if CBD’s effects on FAAH and fatty acid-binding proteins (FABPs) could allow it to raise anandamide (AEA) levels in significant ways (Elmes et al., 2015).
Other molecular targets of CBD
Away from CB1 and CB2, CBD targets a wealth of other receptors, including those belonging to the “expanded ECS”. This more comprehensive system, known as the endocannabinoidome, features a greater scope of receptors, signalling molecules, and enzymes.
Some of CBD’s molecular targets include:
-
TRPV1: Also known as the capsaicin receptor, TRPV1 underpins the burning sensation that accompanies eating chillies. It plays a role in pain perception and helps to regulate body temperature. CBD acts as a TRPV1 agonist, meaning it activates it.
-
Orphan G-coupled protein receptors (GPCRs): These receptors work in a similar manner to classical ECS receptors, but are classed as “orphan” because their endogenous signalling molecules remain unknown. CBD works as an agonist at GPR18, a site involved in inflammation and pain, and an antagonist (a substance that stops a receptor from producing a response) at GPR55—a receptor that plays a role in anxiety and stress response (Shi et al., 2017).
-
Peroxisome proliferator-activated receptors (PPARs): These receptors are located on the membrane of cell nuclei and play a major role in gene expression and fatty acid metabolism. Out of the three PPARs, CBD binds to PPARy, which plays a regulatory role in metabolism.
-
Serotonin receptors: CBD also binds to the serotonin receptor 5HT1A, a site involved in the mechanism of anti-anxiety, antidepressant, and antipsychotic medications.
-
GABA receptors: Gamma-aminobutyric acid (GABA) receptors play an important role in suppressing brain signalling. CBD works as an agonist of the GABA-A receptor.
What CBD products are there?
Since CBD skyrocketed in popularity around 2014, the market has become saturated with products. Nowadays, you’ll find CBD in various manifestations lining the shelves of supermarkets, petrol stations, and even pet stores. Everything from CBD oil and capsules to cosmetics, creams, drinks, sweets, and even dog treats can be purchased. Below, we’ll explore two of the most popular ways to take CBD: CBD oil and CBD capsules.
CBD oil

CBD oil is the mainstay of the CBD market. Offering ease, simplicity, and portability, most CBD oils feature CBD extract suspended in an edible carrier oil, such as olive oil, hemp seed oil, or MCT oil. These substances serve as a lipid-rich base that works to distribute and carry fat-loving CBD molecules. While the majority of CBD oils share many similarities, they can differ in chemical composition. Generally, CBD oils fall into one of the following three categories.
🔹Full spectrum
Full-spectrum CBD oil offers perhaps the most accurate representation of the hemp phytocomplex. Put simply, full-spectrum CBD products contain a diverse array of cannabis constituents. Though CBD features in the greatest concentration, varying amounts of other cannabinoids, terpenes, and trace substances are also included—including THC. Note, however, that THC exists in such small concentrations that it is not capable of causing intoxication.
Emerging research suggests that full-spectrum CBD products capitalise on the “entourage effect”, which posits that cannabis phytochemicals produce synergistic effects when taken together.
🔹Broad spectrum
Broad-spectrum CBD oils differ from full-spectrum in that they contain no THC whatsoever. They still contain other minor cannabinoids, terpenes, etc., but the complete removal of THC eliminates the possibility of any issues associated with the psychotropic cannabinoid.
🔹Isolate
CBD oils made from CBD isolate contain around 99% cannabidiol. Manufacturers use processes that strip everything else away, leaving only pure CBD crystals behind. These crystals are then infused into a carrier oil. Therefore, isolate CBD oils offer higher concentrations of CBD than other oils, but lack the potential benefits of the entourage effect.
CBD oil: sublingual vs oral
One of the reasons CBD oil is so popular is its versatility. It can be taken sublingually (under the tongue) or orally (swallowed as is, or taken in food or drink).
Taken orally, the compound must first contend with the digestive system and liver before it reaches systemic circulation. As a result, over half of the CBD is lost. However, once the effects take hold, they tend to last longer compared to sublingual intake.
Sublingual administration allows CBD to diffuse into the capillaries on the floor of the mouth, offering the cannabinoid near-instant access to the bloodstream. Therefore, the sublingual route offers a faster onset and improved absorption, but potentially shorter-lasting effects.
CBD capsules

CBD capsules are simply encapsulated CBD oil. Many producers use softgel capsules, as they are easy to swallow. Each capsule contains a precise dose of CBD, giving the user greater control over how much they take. Furthermore, this method tends to be more discreet than taking CBD oil.
The downside? Capsules aren’t as versatile when it comes to route of administration, and are exclusively for oral consumption. However, that hasn’t stopped some users from biting into them and squeezing oil under their tongue.
What are the risks of hemp-derived CBD?

CBD often gets marketed as a risk-free substance. But there are several factors to keep in mind when it comes to using the cannabinoid safely. The World Health Organization (WHO) has stated that CBD does not appear to have abuse potential or cause harm. As the cannabinoid is non-psychotropic, it does not pose a risk to those susceptible to mental health issues.
However, the cannabinoid is known to produce side effects in some people, such as:
- Dry mouth
- Diarrhoea
- Reduced appetite
- Drowsiness
- Fatigue
These side effects are relatively minor, but the biggest risk involved with taking CBD arises when using it alongside other drugs. CBD strongly inhibits a family of liver enzymes known as cytochrome P450. These proteins are responsible for breaking down the majority of known drugs. Because CBD limits the activity of these enzymes, the molecule also inhibits the metabolism of other drugs. Therefore, those taking any form of medication should always consult with their physician before taking CBD.
CBD vs THC
The key differences between CBD and THC boil down to molecular structure and mechanism of action. THC’s structure allows it to bind directly to CB1 receptors of the ECS, causing an acute surge of dopamine and other chemical cascades that culminate in the cannabis “high”. Rather than binding to CB1, CBD changes the way THC interacts with the receptor. Many cannabis users find that taking THC and CBD together results in a more balanced experience.
Why is CBD so popular?

Scientists discovered CBD over eighty years ago. So why are people just now becoming excited about the cannabinoid? Hemp-derived cannabidiol has emerged as a mighty force within the dietary supplements industry, and for good reason. Preclinical research has discovered some interesting mechanisms of action and areas of clinical potential that make CBD a deserving target of more significant research.
However, comprehensive clinical trials are needed to determine if and how CBD might benefit particular conditions. It’s important to look past the hype and recognise that we still know so little about this cannabinoid and its widespread effects on human physiology.





 United States
United States











